A Food and Drug Administration (FDA) draft guidance would require manufacturers of pulse oximeters to gather far more clinical data to show the devices accurately work across a range of skin tones.
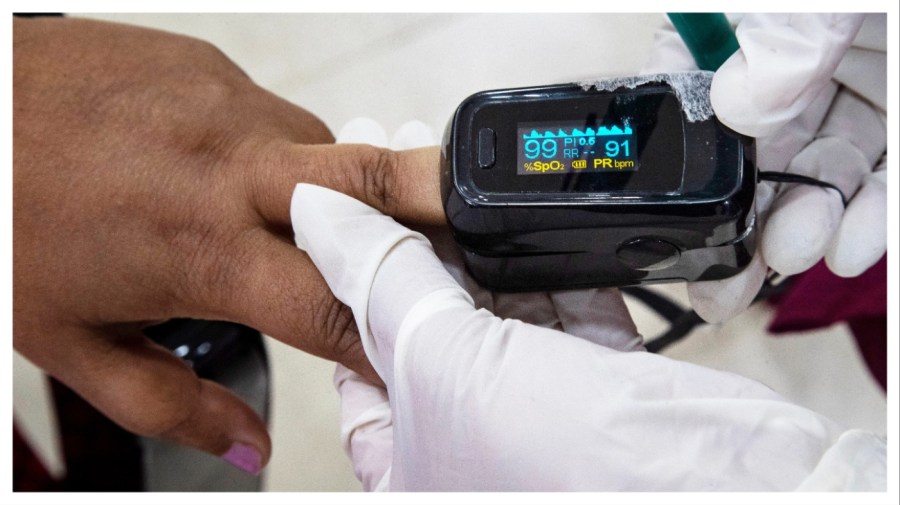
The long-awaited guidance released Monday comes in response to mounting evidence that the devices, which are used to quickly estimate a person’s blood oxygen levels, are less accurate on people with darker skin.
The FDA said it wants companies to test their devices on at least 150 people with a wider variety of skin tones, including 25 percent of participants with light, medium and dark skin tones. Half of the participants in the dark-skin tone should have very dark skin, the agency recommended.
FDA also said companies should evaluate the pigmentation of study participants using at least two different methods, including a subjective visual assessment from the researcher conducting the trial, and a scientific-based measurement.
The agency said it will take public comments on its proposal for 60 days before beginning work on a final version.
The draft guidance is aimed at reducing racial disparities in how well pulse oximeters work.
Pulse oximeters clip on to a person’s finger and use two wavelengths of light sent into the skin to quickly estimate how much oxygen is flowing through the blood based on how much light is absorbed.
The devices became a mainstay of emergency care during the COVID-19 pandemic. But studies dating back decades have suggested that the devices don’t work as well on patients with darker skin pigmentation.
FDA warned doctors and the public about the device’s limitations in a 2021 safety communication.
Melanin absorbs light, so the darker a person’s skin, the less light from the device passes through. Newer research has shown that pulse oximeters overestimate Black patients’ oxygen levels, which could lead to delays in getting treatment and increased risks of death, especially due to COVID-19.
The draft guidance is not legally binding and applies only to pulse oximeters used in hospitals and other medical settings. There are numerous devices sold over the counter, but FDA doesn’t regulate them.